Disclosure of Invention
The purpose of the invention is as follows: the invention aims to provide a preparation method of a micro hydrogel with colon-specific delivery.
The technical scheme is as follows: the invention provides a preparation method of a micro hydrogel with colon-specific delivery, which comprises the following steps:
(1) placing puerarin in sterile distilled water, stirring to completely dissolve to obtain puerarin solution, then placing low methoxyl pectin in puerarin solution, stirring to hydrate to obtain puerarin-containing low methoxyl pectin water solution, standing to remove bubbles;
(2) adding span 80 into mineral oil, stirring, and standing overnight to remove bubbles;
(3) adding chitosan into an aqueous solution containing glacial acetic acid, stirring until the chitosan is completely dissolved, and adding a cross-linking agent to prepare a chitosan solution containing zinc acetate;
(4) sucking the puerarin-containing low methoxyl pectin aqueous solution obtained in the step (1) into a water phase sample injector of a microfluidic system, sucking the mineral oil obtained in the step (2) into an oil phase sample injector of the microfluidic system, and regulating the particle size of the micro hydrogel by controlling the speed of the water phase and the oil phase;
(5) and (4) dropwise adding the water-in-oil solution obtained in the step (4) into the chitosan solution obtained in the step (3), and stirring at a low speed to prepare the puerarin-containing pectin-chitosan-cross-linking agent micro hydrogel.
Further, the cross-linking agent is zinc acetate.
Further, the main structure of the chip in the microfluidic system comprises an upper layer substrate and a lower layer substrate.
Furthermore, the upper and lower substrates are made of polydimethylsiloxane and glass materials respectively.
Further, the concentration of the chitosan is 0.2%, 0.4% or 0.6%.
Further, the concentration of the low methoxyl pectin aqueous solution is 0.5%, 1%, 2%, 4% or 6%.
Further, the micro-hydrogel delivers puerarin specifically at the colon site.
Has the advantages that: (1) the pectin and the chitosan are used as main raw materials, are natural polysaccharides, have good biocompatibility and degradability, and can meet the requirements of oral materials; (2) pectin and chitosan are resistant to enzymes in the stomach and intestinal tract, but are specifically degraded by enzymes produced by the colonic flora, making pectin and chitosan based delivery systems the most suitable choice for colon specific delivery; (3) the invention adopts the microfluid technology and provides a new innovative method for the preparation and research of the hydrogel. The prepared micro hydrogel with uniform particle size stably exists in the gastrointestinal tract and is slowly degraded in the colon, so that the micro hydrogel is the best choice for colon-specific delivery; (4) the invention loads and releases puerarin with anti-colon cancer effect, and the result shows that the micro hydrogel not only has slow release effect, but also can reduce the inhibition effect of the puerarin on the enzymatic activity of protease with digestive effect in gastrointestinal tract.
Detailed Description
Experimental materials:
puerarin is purchased from energy chemical industry (purity 98%; Shanghai, China). Food-grade low-methoxyl pectin (dry basis is more than or equal to 74.0 percent), sorbitan monooleate (CAS: 1338-43-8), paraffin liquid (CAS: 8012-95-1), pectinase (CAS: 9032-75-1, 30000U/g) and chitosan (more than or equal to 95 percent deacetylation, viscosity 100-. Zinc acetate was purchased from alatin (99% pure; shanghai, china). Chitosanase was purchased from Yuye Biotechnology, Inc. (Shanghai). Dimethyl sulfoxide (DMSO), 3- (4, 5-dimethyl-2-thiazolyl) -2, 5-diphenyl-2H-tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St. Louis, Mo.).
Example 1: functional activity, biocompatibility and influence on protease structure of puerarin.
(1) Colon cancer cells (HCT116 cells) in logarithmic growth phase were digested, counted, adjusted to initial cell concentrations of 1000/well and 2000/well, seeded into 96-well plates, respectively, and placed in 5% CO2Culturing in incubator for 24 hr, preparing puerarin into 25, 50, 100, 200, 400, 800 μ g/mL solution, and filtering with 0.22 μm water system filter membrane. And (3) sucking out the cell supernatant culture solution of the 96-well plate by using a pipette gun, adding the puerarin solution into a blank group which is a blank complete culture medium without the puerarin solution, continuously culturing the cells, and detecting the cell activity by using an MTT method after culturing for 72h and 48h respectively. The obtained cell survival rates are respectively shown in (a) and (b) in fig. 2, wherein the results of the (a) graph show that the initial concentration of HCT116 cells is 1000/hole, and when the cells are cultured for 72 hours, the higher the puerarin concentration is, the higher the cell inhibition rate is, so that the puerarin has a better colon cancer inhibition effect; (b) the results of the graphs show that the initial concentration of HCT116 cells is 2000 cells/hole, and the cell inhibition rate is increased along with the higher concentration of puerarin when the cells are cultured for 48 hours, which indicates that the puerarin also has higher contentGood colon cancer inhibiting effect. Therefore, it can be said that puerarin is a substance having a good colon cancer inhibitory activity.
(2) Collecting RAW264.7 in logarithmic growth phase, counting, and adjusting initial concentration of cells to 50 × 104one/mL, inoculated into 96-well plates, respectively, and placed in 5% CO2Culturing in incubator for 24 hr, preparing puerarin into 25, 50, 100, 200, 400, 800 μ g/mL solution, and filtering with 0.22 μm water system filter membrane. And (3) sucking out the cell supernatant culture solution of the 96-well plate by using a pipette gun, adding the puerarin solution into the culture solution, continuously culturing the cells by using a blank complete culture medium without the puerarin solution, and detecting the cell activity by using an MTT (methanol to transfer) method after culturing for 24 hours to obtain the cell survival rate. As shown in (c) and (d) of FIG. 2, the results of (c) are that the cell viability under the action of high concentration puerarin is higher than 90% at the absorbance value of 490nm of the detection wavelength, which indicates that puerarin has good biocompatibility; (d) the results of the graphs show that the cell survival rate under the action of high-concentration puerarin is similar to that of a blank control group without puerarin and is higher than 90 percent at the light absorption value of reference wavelength of 570nm, which indicates that the puerarin has good biocompatibility.
(3) Trypsin solution (2.0mL, 0.2mM) was added to each 5mL centrifuge tube at room temperature, and then 0, 10, 20, 30, 40 and 50. mu. L1.0mM puerarin solutions were added to the centrifuge tubes. After adding puerarin solution, mixing the mixture uniformly, and scanning absorption spectrum at 200nm-350nm with ultraviolet spectrophotometer after 2 minutes to obtain ultraviolet absorption difference spectrum of trypsin under action of puerarin with gradient concentration as shown in (a) in figure 3. And the emission spectrum of trypsin at 300-450nm under the action of puerarin with gradient concentration as shown in (c) in FIG. 3 is obtained by detecting with a fluorescence spectrophotometer under the excitation wavelength of λ ex-295 nm. Similar to the trypsin assay, the pepsin solution (2.0mL, 0.5X 10) was aspirated-2mM) was placed in a centrifuge tube, followed by addition of puerarin solution and uniform mixing. Scanning the absorption spectrum at 200-300nm with a UV spectrophotometer after 2 minutes to obtain the UV absorption difference spectrum of pepsin under the action of graded concentration puerarin as shown in (b) in FIG. 3, and obtaining the absorption difference spectrum at λ ex ═ 295 and λ ex-Fluorescence emission spectra of pepsin at 300-450nm under the action of graded concentration puerarin are respectively detected as (d) and (e) in fig. 3 at an excitation wavelength of 278 nm. The ultraviolet absorption spectrum and fluorescence spectrum results show that puerarin has influence on the space structures of trypsin and pepsin, and the influence is more obvious as the concentration of the puerarin is increased.
Example 2: preparation of the micro-hydrogels
(1) Puerarin was put in sterile distilled water and stirred until completely dissolved to prepare a puerarin solution (800. mu.g/mL). Then, adding low methoxyl pectin into puerarin solution, stirring until completely hydrated to prepare continuous phase, and standing to remove bubbles after low methoxyl pectin is completely hydrated. The chitosan solution was prepared by placing chitosan in an acetic acid solution (1% w/v) to dissolve it completely. Zinc acetate was added to the chitosan solution and stirred for 2 hours to prepare a chitosan solution containing zinc acetate (5% w/v). Sorbitol monooleate (1% w/v) was added to liquid paraffin and stirred for 2 hours, and then left overnight to remove air bubbles for preparing a dispersed phase. All experiments were performed at room temperature.
(2) The continuous phase and the dispersed phase are injected into the microfluidic chip by using a pressure system, the flow is controlled by using a flow sensor, the flow sensor is connected onto the chip by a syringe and a fused silica capillary, and the size of the gel can be adjusted by controlling the speed of the continuous phase and the dispersed phase. Unless otherwise stated, the continuous phase flow rate was 2. mu.L/min, the dispersed phase flow rate was 20. mu.L/min, and the resulting droplet diameter was about 100. mu.m. The chip holder was used to place the chip under an optical microscope. Images were recorded at the inlet and outlet of the cruciform junction, coalescence channel. (the micro-fluidic system comprises a dispersed phase inlet 1, a first continuous phase inlet 2, a second continuous phase inlet 3, a third continuous phase inlet 4, a cross-shaped junction intersection 5 of the dispersed phase and the continuous phase, a merging channel 6, a magnetic stirrer and a beaker 7 arranged on the magnetic stirrer, wherein the dispersed phase cuts the continuous phase to form water-in-oil droplets for collecting samples, the beaker 7 is internally added with a zinc acetate-chitosan solution, and the droplets prepared by the micro-fluidic system are collected and stirred at low speed under the magnetic stirrer so that the droplets are demulsified and then are subjected to electrostatic crosslinking to form micro-hydrogel. the micro-hydrogel is shown in figure 1)
(3) Aqueous solutions of low methoxyl pectin (0.5%, 1%, 2%, 4% and 6%) at different concentrations were cross-linked with chitosan solution containing zinc acetate to form samples, and the resulting particles were washed repeatedly with distilled water until no liquid paraffin was present, and the washed samples were diluted with water. A small amount of sample was pipetted onto the slide, carefully covered with a cover slip, and the morphology of the hydrogel was observed using an inverted microscope equipped with a digital camera. As shown in fig. 4 (a), (b), (c), (d) and (e), 0.5%, 1%, 2%, 4% and 6% of low methoxyl pectin and chitosan solution containing zinc acetate are crosslinked to form micro hydrogel. By comparing the morphology of the micro-hydrogels, the micro-hydrogel morphology of panel (e) was found to be the most stable, thereby determining the optimal pectin concentration value for forming the micro-hydrogels to be 6%. The effect of zinc acetate on the crosslinking result was investigated by crosslinking pectin at optimum concentration with a chitosan solution without zinc acetate. The results show that a morphologically stable micro hydrogel could not be formed, as shown in figure (f). The results show that a 6% pectin solution and a chitosan solution containing zinc acetate can form a stable micro hydrogel.
(4) Different concentrations of chitin solution (0.2%, 0.4% and 0.6%) coated hydrogels were immersed in NaCl solution (0.9%) at 37 ℃ at a rate of 150r/min, the hydrogels were observed microscopically every 1 hour, and size measurements were performed on at least 60 particles using Nano Measurer 1.2 software. By comparing the swelling ratios of the different chitosan-coated micro hydrogels in the range of 37 ℃, the results are shown in fig. 5 (a), and the optimal chitosan concentration of the micro hydrogel is determined to be 0.6%.
(5) Zeta potentials of the hydrogel and the puerarin-loaded hydrogel were measured using a Zetasizer Nano-ZS90 instrument over a pH range of 2.0-11.0, as shown in fig. 5 (d), indicating that the surface of the micro-hydrogel was positively charged. The particle size distributions of the unloaded and puerarin loaded micro hydrogel particles were measured using Nano Measurer 1.2 software, as shown in (b) and (c) of fig. 5, and the results indicate that the particle size of the puerarin loaded micro hydrogel was significantly larger than the unloaded micro hydrogel.
Example 3: research on encapsulation efficiency and stability of hydrogel on puerarin
(1) A standard curve was established using a puerarin standard by High Performance Liquid Chromatography (HPLC), and as shown in fig. 6 (a), it is a liquid chromatogram of the puerarin standard. Pectinase and chitosanase hydrolyze the blank control group and the puerarin-loaded micro hydrogel sample group, and (b) and (c) in fig. 6 are liquid chromatograms of the blank control group and the puerarin-loaded group, respectively. After the hydrogel was completely hydrolyzed, it was filtered through a 0.22 μm filter, and the supernatant was subjected to high performance liquid chromatography to detect the content of puerarin. In the present embodiment, a tube with Tuna C18(2)
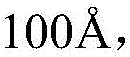
RP-HPLC system (Agilent1200, Shanghai, China) with a 250X4.6mm column. The column temperature used was 25 ℃ and the ratio of mobile phase methanol to water was 1: 3. The detection wavelength was set at 250nm and the flow rate was 1 mL/min. The sample size was set to 20 μ L. As shown in fig. 6 (d), the result showed that the encapsulation efficiency of the micro hydrogel to puerarin was 54.1%.
(2) To investigate the enzymatic properties of the prepared micro-hydrogels, in vitro release experiments were performed at 37 ℃. The same mass of puerarin loaded micro-hydrogels were incubated in normal saline (0.9% NaCl), simulated gastric fluid (pH 2.2), simulated intestinal fluid (pH 7.2) and simulated colon fluid (containing pectinase and chitosanase) for 6 hours to study the release of the micro-hydrogels in different media, and the puerarin content in the supernatant was determined by RP-HPLC every hour, and each experiment was repeated at least three times. As shown in (a), (b), (c) and (d) of fig. 7, the results indicate that the puerarin-loaded micro hydrogel is relatively stable in physiological saline, simulated gastric fluid and simulated intestinal fluid, and releases a large amount of puerarin in colonic fluid.
(3) To simulate the digestion conditions of the gastrointestinal tract, the puerarin loaded micro-hydrogel was first placed in citric acid buffer (pH 2.2) for 2h to simulate gastric digestion, then transferred to citric acid buffer (pH 7.2) for 6h to simulate small intestine digestion, and finally the pectinase and chitosanase lysates were transferred to citrate buffer for 2h to simulate colonic digestion. The micro-hydrogel was continuously stirred on a constant temperature shaker at 150 rpm. Puerarin release was measured by RP-HPLC for each system every 1 hour, and each experiment was repeated at least three times. As shown in fig. 7 (e), the results showed that puerarin was released in small amounts in stomach and small intestine fluids, and in colon fluids, puerarin was released rapidly in the first hour and then slowly.
Example 4: structural characterization of hydrogels
(1) Fourier Infrared Spectroscopy
A Fourier transform infrared spectrophotometer is used for researching the existence state of puerarin in the pectin-chitosan-zinc acetate micron hydrogel and the interaction force of each component.
The components used for the experiment were prepared by freeze-drying, and the lyophilization process of the micro-hydrogels was performed in a Christ Alpha1-4 lyophilizer (Christ, ALPHA 1-4/2-4LD plus, Shanghai). At 400 to 4000cm-1The FT-IR spectra of puerarin, unloaded hydrogel and loaded hydrogel were obtained in the wavenumber range of (a), and from the results shown in fig. 7, the infrared spectrum of the puerarin-loaded hydrogel did not include the absorption peak of puerarin and coincided with the absorption peak of the unloaded hydrogel, as shown in (a) in fig. 8, it was revealed that puerarin existed in the network structure of the hydrogel in a free state.
To verify the structure of the micro-hydrogels, the thickness of the micro-hydrogels was between 400 and 4000cm-1The FT-IR spectra of the components were obtained in the wavenumber range of (a) to provide information on the nature of the chemical bonds involved in the formation of the micro-hydrogel. As shown in FIG. 7 (b), at 1745cm-1And 1648cm-1The absorption peak is the stretching vibration peak of methoxyl and carboxyl in pectin. The stretching vibration peaks of the amido group, amido bond and amino group of the chitosan are respectively 1554cm-1、1632cm-1And 3216cm-1Is absorbed by the absorption body. At 1553cm-1And 1453cm-1The absorption bands at (a) represent the symmetric and asymmetric tensile vibrations of the carboxyl group in zinc acetate. In this study, the condensation reaction of the amino groups in chitosan with the carboxyl groups in pectin indicated that acetate stabilized water as a cross-linking agent when zinc cross-linkedThe effect of the gel, zinc acetate, is similar to the effect of calcium ions in chitosan/sodium alginate gel. By comparing the Fourier transform infrared spectra of pectin, chitosan, zinc acetate and hydrogel, it was found that the hydrogel spectrum showed 1755cm of pectin-1Stretching vibration peak of methoxy group (2). At 1100--1Nearby a wider absorption band, 1553cm of amine group in zinc can be seen-1Stretching vibration peak and 531cm in zinc acetate-1Zn-O stretching vibration peak of (1). Related to the tensile vibration of CN. As shown in FIG. 8 (b), it was confirmed that the micro hydrogel was formed by electrostatic crosslinking.
(2) Analysis of thermal Properties
Thermogravimetry (TGA) has been widely used to confirm the detected change in thermal stability of hydrogels with temperature. For TGA analysis, hydrogels prepared by microfluidic techniques were placed in centrifuge tubes and centrifuged to remove distilled water and used for experiments. Stability and thermal degradation of the puerarin loaded micro-hydrogels were evaluated using a thermogravimetric analyzer TGA-50 (Shimadzu Corporation, kyoto, japan). First, a sample (10mg) was heated in a platinum pan at a rate of 10 ℃/min under a stream of nitrogen at 40mL/min and analyzed at a temperature of 30 to 150 ℃.
The results show that the weight of the micro hydrogel consisting of pectin-chitosan-zinc acetate decreases with increasing temperature. As shown in fig. 8 (c), when the temperature was increased from 32 ℃ to 120 ℃, the weight changed significantly, and after 120 ℃, the weight hardly changed any more, and the weight loss in this temperature range corresponded to the evaporation of free water, as shown in fig. 8 (c), indicating that the free water content in the micro hydrogel was 94.36%.
(4) Transmission electron microscope
Transmission Electron Microscope (TEM) imaging can provide a great deal of information for hydrogels. The structure of the hydrogel was observed using a CM10 transmission electron microscope (HITACHI, H-9500, Japan). Freshly prepared hydrogels (10 μ L) were deposited on a 3.05mm grid using ultrathin sections (80-100nm gold and silver) and excess water was absorbed with filter paper and air dried for observation. As shown in FIG. 9(a), the micro hydrogels were all spherical structures with more uniform shape and size, as shown in FIG. 9(b), indicating that the micro hydrogels had a dense core and a thin coating. Fig. 9(c) shows that the chitosan-zinc acetate composite coating may be surface polymer chains extending into the aqueous phase and may be considered as a halo of light around the particles. As shown in fig. 9 (d), after digestion in simulated colonic fluid, the spherical micro-hydrogel was hydrolyzed by colonic enzymes. The particle sizes measured by TEM are all smaller than the hydrodynamic particle size collected by dynamic light scattering due to the drying process of TEM sample preparation.